Your preferred partner in the field of medical products
Consulting
Service
Training
We advise and support you from the idea to approval.
This includes the preparation of technical documentation and the development of QM systems in accordance with ISO 13485. We also support and advise you in the area of post-market surveillance, take on the tasks of the PRRC (MDR Art. 15) and prepare you for audits.
We offer you everything from individual consulting services to an all-round carefree package.
Consulting services
We offer you individual advice and support.
Quality management systems for manufacturers (ISO 13485)
Implementation of the Medical Device Regulation (MDR)
Risk management for medical devices
(ISO 14971)
Programmable Electrical Medical Systems (PEMS)
Software life cycle
(IEC 62304)
Usability-oriented development process
(IEC 62366-1)
Electrical safety of medical devices
(ISO 60601-1)
Technical documentation for medical devices according to normative requirements
Services
The following Services we can take over for you.

Audit service
We carry out the required internal system audits (in accordance with ISO 13485) for you.

PRRC service
We take over the tasks of the responsible person for your company in accordance with Art. 15, provided it is a micro or small enterprise as defined in the recommendation under 2003/361/EC.

EU Rep Service
We assume the mandate as authorized representative in accordance with Art. 11. and act as the interface between you and the authorities if you are based outside the EU.

Implementation service
We support you with the implementation of a QMS in accordance with ISO 13485 and accompany you until you receive the certificate.
Our training courses for you
Complete all four courses and, after successfully passing the exam, you will receive your own certificate as a Manager Regulatory Affairs.
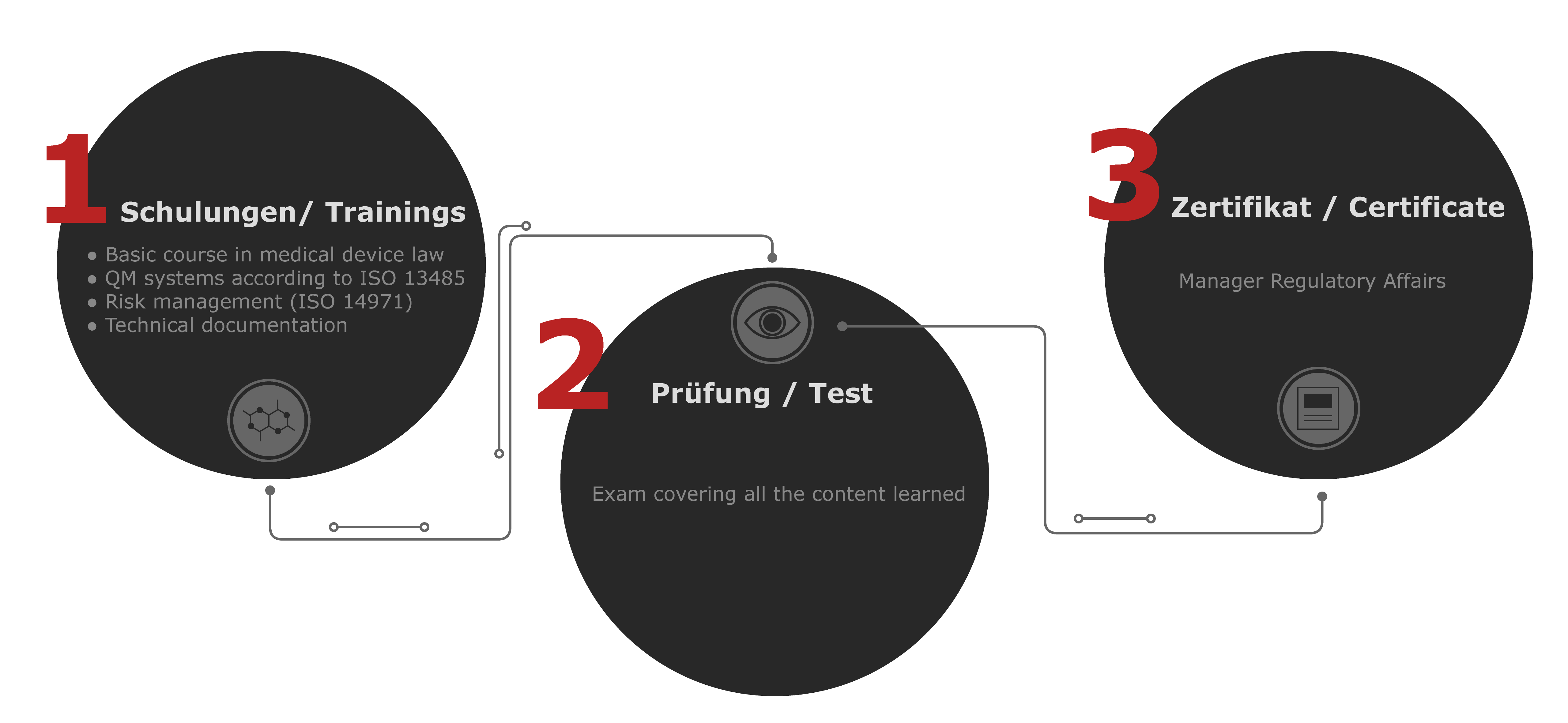
Basic course on medical device law MDR
€ 490
/ 1 day
- Current legal framework for medical devices in Europe (MDR, 2017/745)
- Classification of medical devices, conformity assessment procedures, CE marking
- Basic safety and performance requirements
Quality management systems for manufacturers according to ISO 13485
€ 490
/ 1 day
- Structure, content and application of ISO 13485:2016
- Consolidation of selected core processes
Risk management for medical devices according to ISO 14971
€ 690
/ 2 days
- Requirements of ISO 14971 and their implementation
- Risk management plan, risk analysis, risk assessment
- Practice-oriented implementation workshop
Technical documentation for medical devices
€ 490
/ 1 day
- Requirements, contents of technical documentation
- Significance for conformity assessment procedures, notified bodies and authorities
- Basics of clinical evaluation
- Basics of post-market surveillance
Frequently asked questions
Yes, at least five participants must be registered for a course to take place.
The training courses can be held in-house, either online or in person, if the hygiene measures ensure this.
In-house training is an instrument of company training in the context of personnel development. Employees receive a customized in-house course from our consultants.
The training courses are held by our consultants.

